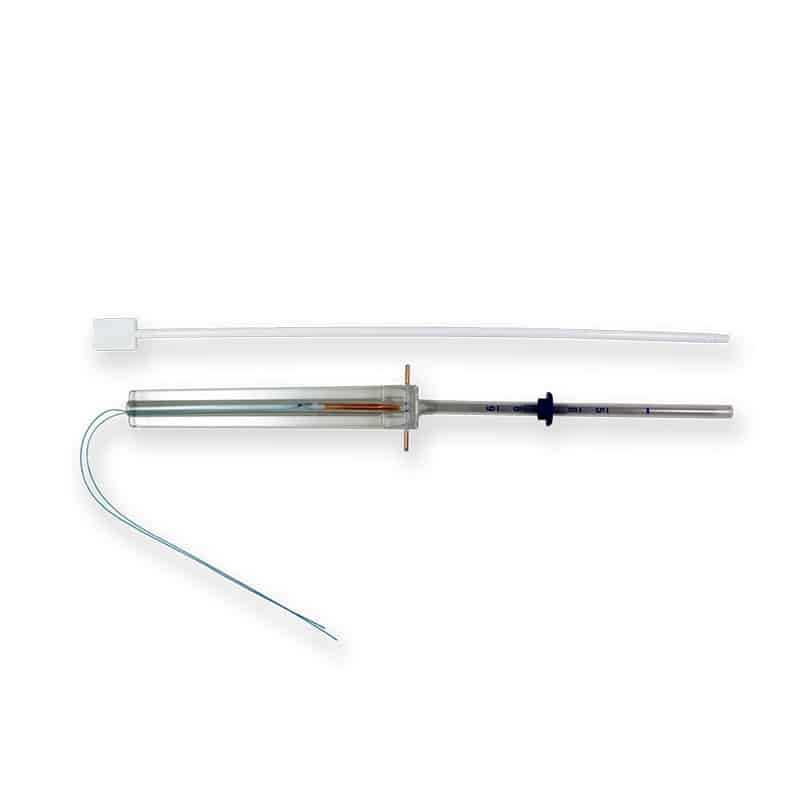
Product Description
The T-SAFE® CU 380A QL IUD is a highly reliable, hormone-free intrauterine device (IUD) used for long-term contraception. This summary explores key aspects such as its indications for use, effectiveness, side effects, clinical outcomes, and other critical details.
T-SAFE® CU 380A QL IUD Indications for Use
The T-SAFE® CU 380A QL IUD is primarily designed for women seeking a long-term, hormone-free contraception solution. It is recommended for women of reproductive age, including those who prefer non-hormonal options or are sensitive to hormonal contraception. The IUD is ideal for:
- Women who have given birth: It is often recommended for women who have already given birth as their uterus size typically fits the standard IUD dimensions.
- Women seeking long-term contraception: The T-SAFE® CU 380A can remain in the uterus for up to 10 years, offering extended contraceptive protection.
This IUD is inserted into the uterus by a healthcare provider and works immediately after insertion.
T-SAFE® CU 380A QL IUD Mechanism of Action
The T-SAFE® CU 380A QL relies on copper ions released from the copper coil wrapped around the device. These copper ions interfere with sperm motility and their ability to fertilize an egg. Additionally, the presence of the IUD in the uterus causes a foreign body reaction, altering the uterine lining in ways that further inhibit implantation.
- Copper ions: Decrease sperm mobility and prevent fertilization.
- Foreign body reaction: Alters uterine lining to reduce chances of embryo implantation.
This dual-action makes the T-SAFE® CU 380A a highly effective contraceptive method.
T-SAFE® CU 380A QL IUD Dosage Information and Longevity
The T-SAFE® CU 380A QL contains 380 square millimeters of copper. It is designed to remain in the uterus for a maximum of 10 years, making it one of the longest-lasting IUDs available.
- Recommended uterus length: 6-9 cm.
- Effective lifespan: 10 years in situ.
After the recommended duration, the device must be replaced or removed by a healthcare provider.
T-SAFE® CU 380A QL IUD Side Effects and Precautions
While the T-SAFE® CU 380A is generally well-tolerated, there are several possible side effects, particularly during the first few months after insertion:
- Mild cramps: Common in the initial days following insertion.
- Prolonged menstrual bleeding: Can occur in the first 2-3 months.
- Additional bleeding or spotting: Some users experience unplanned bleeding.
- Dizziness: A few women may report slight dizziness after insertion.
If any side effects become severe or persistent, consulting a healthcare provider is essential. Most of these symptoms subside as the body adjusts to the device.
T-SAFE® CU 380A QL IUD Insertion and Removal
Insertion of the T-SAFE® CU 380A QL is typically performed during menstruation when the cervix is slightly more open, making the process easier. However, the device can be inserted at any point in the menstrual cycle if pregnancy is ruled out.
- Emergency contraception: The IUD can also be used as emergency contraception if inserted within 5 days of unprotected sex.
Removal is straightforward and can be performed at any time by a trained professional. After removal, women can expect to return to normal fertility immediately.
T-SAFE® CU 380A QL IUD Clinical Studies and Real-World Outcomes
The T-SAFE® CU 380A QL has demonstrated excellent efficacy in clinical trials and real-world use. Its effectiveness is comparable to other long-term contraceptive methods such as hormonal IUDs or implants.
- Pregnancy rate: Fewer than 1% of women using the device for the full 10 years will experience an unintended pregnancy, making it a highly reliable contraceptive option.
Clinical studies also indicate that the device does not interfere with a woman’s hormonal balance, contributing to its popularity among those preferring hormone-free solutions.
T-SAFE® CU 380A QL IUD Contraindications and Precautions
Like any medical device, the T-SAFE® CU 380A QL has certain contraindications. Women with the following conditions should avoid using it:
- Active pelvic infections: These should be treated before considering an IUD.
- Uterine abnormalities: Any malformations of the uterus may interfere with the proper placement or effectiveness of the IUD.
- Copper allergies: Rare, but some women may have an allergy to copper, in which case they should seek alternative contraceptive options.
T-SAFE® CU 380A QL IUD Drug Interactions
The T-SAFE® CU 380A QL IUD has no known drug interactions as it functions through the mechanical action of copper ions rather than hormonal pathways. This makes it a suitable contraceptive option for women who are on medications that might interfere with hormonal contraception, including anticonvulsants or antibiotics.
T-SAFE® CU 380A QL IUD Follow-Up and Monitoring
The T-SAFE® CU 380A requires minimal follow-up, though it is advised to check the IUD one month after insertion or following the first menstrual period. After that, semi-annual check-ups are typically sufficient unless clinical symptoms require additional monitoring.
- Initial check: One month after insertion.
- Routine check-ups: Every 6 months thereafter.
Women are also advised to check the position of their IUD strings after each menstrual period to ensure the device is still in place.
T-SAFE® CU 380A QL IUD Key Benefits of T-SAFE® CU 380A QL IUD
- Long-lasting: Effective for up to 10 years.
- Hormone-free: Ideal for women who prefer or require non-hormonal contraception.
- Highly effective: Less than 1% failure rate when used correctly.
- Low maintenance: Requires only periodic check-ups after insertion.
Immediate return to fertility: Fertility is restored immediately after removal.