Buy OZEMPIC® 1mg (Polish) Online
brand:
OZEMPIC®
manufacturer:
Novo Nordisk
active substances:
SEMAGLUTIDE
strength:
1mg
pack size:
1 pen, 4 disposable NovoFine® Plus needles (4 Doses)
OZEMPIC® 1mg (Polish) Indications for Use
Ozempic® is a prescription medication used for managing type 2 diabetes mellitus in adults. It helps lower blood sugar (glucose) levels and is also used to reduce the risk of major cardiovascular events like heart attacks and strokes in adults with both type 2 diabetes and heart disease.
- Primary Use: Controls blood sugar levels in adults with type 2 diabetes.
- Secondary Use: Lowers the risk of cardiovascular complications in patients with heart disease.
- Target Audience: Adults with type 2 diabetes, especially those at risk for cardiovascular disease.
OZEMPIC® 1mg (Polish) Dosage Information
Ozempic® is administered once weekly via subcutaneous injection. The treatment begins with a low initial dose, which gradually increases as the patient’s body adapts to the medication.
- Initial Dose: Start with 0.25 mg once weekly for the first 4 weeks. This starting dose helps reduce the gastrointestinal side effects that can occur when beginning semaglutide treatment.
- Maintenance Dose: After the first 4 weeks, increase the dose to 0.5 mg once weekly. This dose is effective for controlling blood sugar levels in many patients.
- Maximum Dose: If additional glycemic control is needed, the dose may be increased to 1 mg once weekly, under medical supervision.
Patients should inject Ozempic® on the same day each week. The injection can be taken with or without meals and should be injected into the abdomen, thigh, or upper arm. Ozempic® comes in pre-filled pens containing 1 mg of semaglutide per dose.
OZEMPIC® 1mg (Polish) Side Effects and Precautions
Ozempic® can cause a variety of side effects, ranging from mild to serious. Patients should be aware of these potential side effects and follow appropriate precautions during treatment.
- Common Side Effects:
- Nausea, which is usually mild and diminishes over time.
- Diarrhea or constipation.
- Vomiting.
- Headache and tiredness.
- Decreased appetite, which often leads to weight loss.
- Injection site reactions, such as redness or irritation.
- Serious Side Effects:
- Hypoglycemia (low blood sugar), especially when used with other diabetes medications such as insulin or sulfonylureas.
- Pancreatitis: Symptoms include severe abdominal pain that may radiate to the back, which could indicate inflammation of the pancreas.
- Kidney problems: Dehydration caused by vomiting or diarrhea can lead to worsening kidney function, especially in patients with pre-existing kidney conditions.
Precautions:
- Pregnancy: Ozempic® is not recommended during pregnancy due to potential harm to the fetus. Women who are pregnant or planning pregnancy should avoid Ozempic®.
- Allergic Reactions: Patients allergic to semaglutide or any other ingredients in the formulation should not use the medication. Signs of an allergic reaction include swelling, itching, difficulty breathing, or rash.
OZEMPIC® 1mg (Polish) Clinical Studies or Real-World Outcomes
Ozempic® has been evaluated in clinical trials that demonstrate its effectiveness in managing type 2 diabetes and reducing cardiovascular risks.
- Glycemic Control: Clinical trials showed that Ozempic® reduced HbA1c levels by 1.5% to 2% in patients over 26-52 weeks of treatment, offering significant blood sugar control. Many patients achieved target blood sugar levels with regular use of the drug.
- Weight Loss: Patients using Ozempic® often experienced weight loss due to its appetite-suppressing effects. Studies reported average weight reductions of 4-6 kg during treatment.
- Cardiovascular Protection: In patients with type 2 diabetes and cardiovascular disease, Ozempic® reduced the risk of major cardiovascular events, such as heart attacks and strokes, by approximately 26% compared to placebo.
OZEMPIC® 1mg (Polish) Drug Interactions
Ozempic® may interact with other medications, particularly those used to treat diabetes.
- Hypoglycemia Risk: When Ozempic® is used with insulin or sulfonylurea drugs (e.g., glimepiride, glibenclamide), the risk of hypoglycemia increases. Dose adjustments for insulin or sulfonylurea may be necessary to avoid low blood sugar.
- Other Interactions: Patients should inform their healthcare provider about all medications, supplements, or over-the-counter products they are taking to prevent potential drug interactions.
OZEMPIC® 1mg (Polish) Summary of Benefits
Ozempic® provides several significant benefits for adults with type 2 diabetes, especially those with cardiovascular risks.
- Blood Sugar Control: Helps lower HbA1c levels effectively, providing long-term glycemic management.
- Weight Loss: Encourages weight reduction, which is beneficial for patients struggling with obesity-related complications.
- Cardiovascular Protection: Reduces the risk of heart attacks, strokes, and other cardiovascular events in diabetic patients with pre-existing heart disease.
- Once-Weekly Dosing: Convenient dosing schedule, reducing the burden of daily medication administration.
OZEMPIC® 1mg (Polish) Key Ingredients and Mechanism of Action
The active ingredient in Ozempic® is semaglutide, a GLP-1 receptor agonist that mimics the action of glucagon-like peptide-1 (GLP-1), a natural hormone involved in glucose regulation.
- Semaglutide: Helps increase insulin production in response to high blood sugar, reduces the amount of glucose produced by the liver, and slows down gastric emptying. These actions help to lower blood sugar levels and promote weight loss.
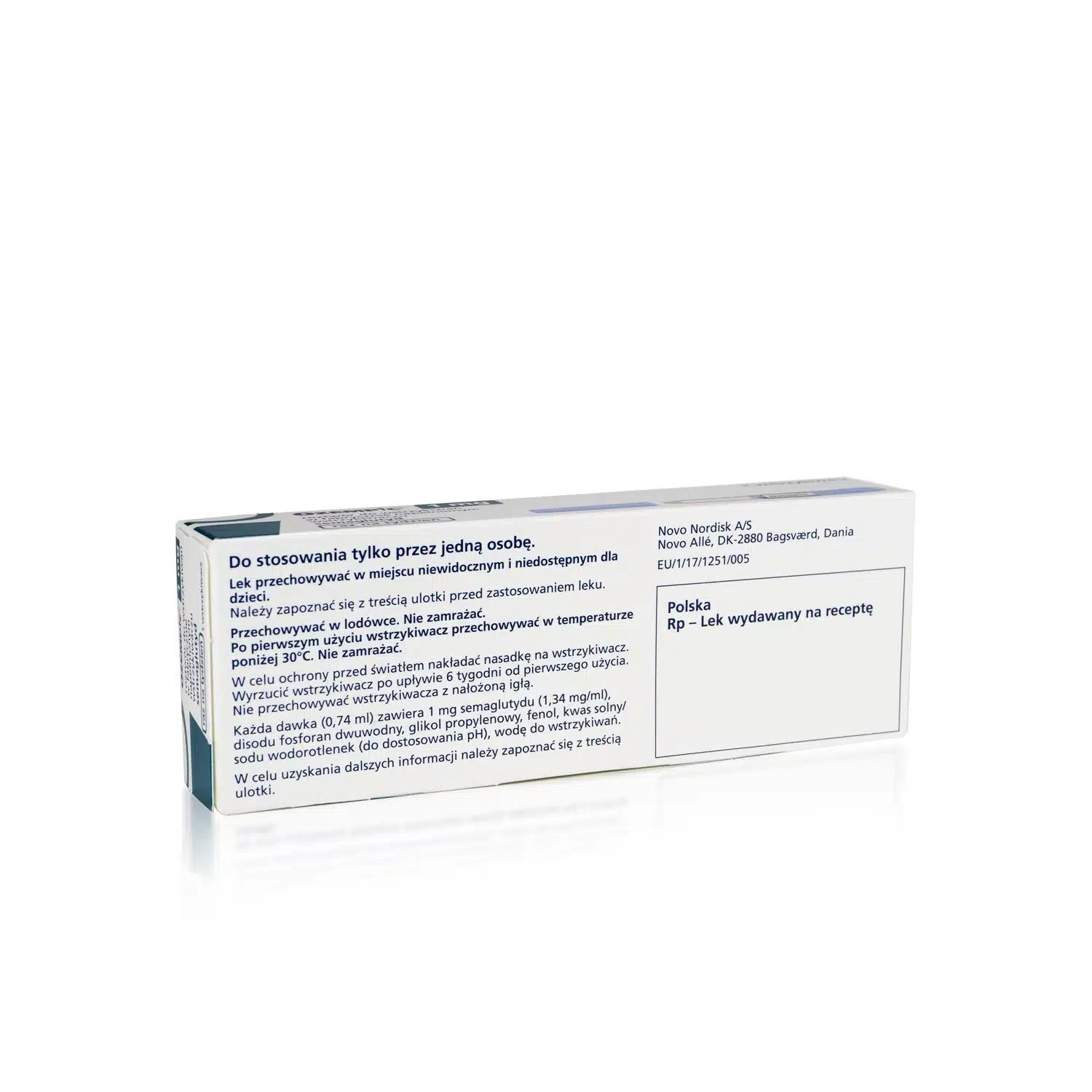
- Inactive Ingredients: Includes dibasic sodium phosphate, propylene glycol, phenol, and water for injection, all of which help stabilize the solution for safe use.
OZEMPIC® 1mg (Polish) Recommended Treatment Protocol
Ozempic® should be integrated into a comprehensive diabetes management plan, including a balanced diet and regular exercise.
- Start with 0.25 mg once weekly for the first 4 weeks to minimize gastrointestinal side effects.
- Increase to 0.5 mg once weekly after 4 weeks for continued blood sugar control.
- Consider increasing to 1 mg weekly if further glucose reduction is necessary.
Patients must adhere to their dosing schedule and administer the medication as directed by their healthcare provider.